
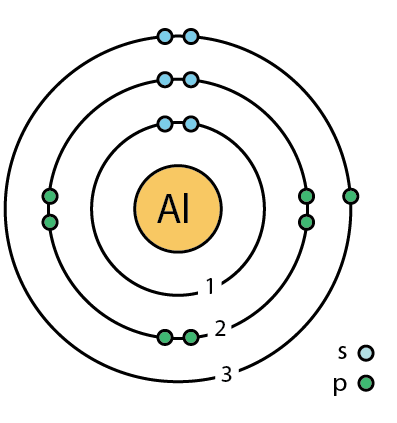
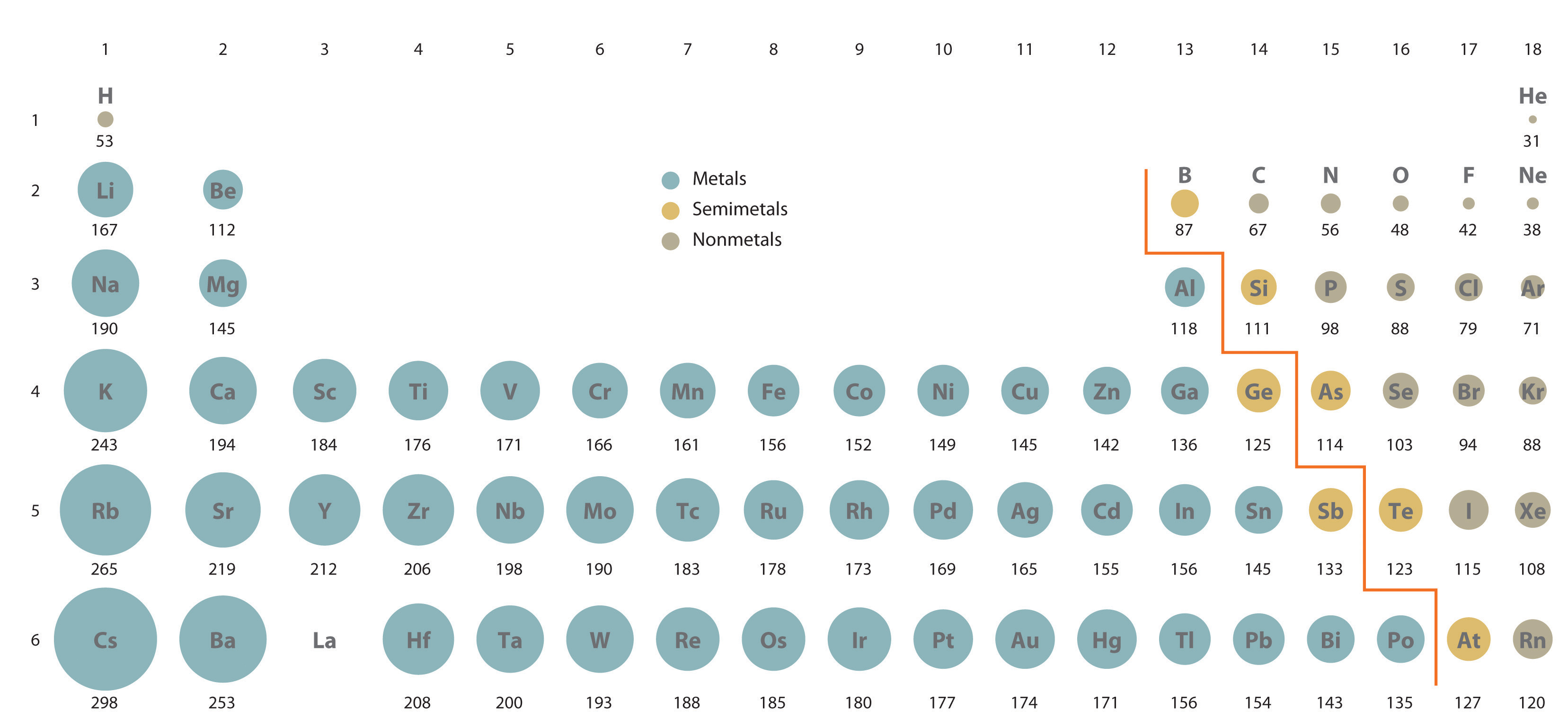
Aluminium, gallium, indium, and thallium have three electrons in their outermost shell (a full s orbital and one electron in the p orbital) with the valence electron configuration $ns^2np^1$. Note: The boron family contains elements in group 13 of the periodic table and include the semi-metal boron (B) and the metals aluminium (Al), gallium (Ga), indium (In), and thallium (Tl). Hence the correct option is (C) B < Ga < Al < In < Tl. Thus, the increasing order of atomic radii of group 13 elements is B < Ga < Al < In < Tl. As a result, the electrons in Ga experience greater force of attraction by the nucleus than in Al and hence the atomic radius of Ga 135 pm is slightly less than that of Al 143 pm. This is due to the presence of d - electrons in Ga which do not shield the nucleus effectively. On moving down the group the atomic radius of Ga is slightly lower than that of Al. Generally, down the group the atomic size increases because new shells add at each step and effectively nuclear charge decreases.Ītomic and ionic radii of group 13 elements are lower than those of alkaline earth metals of group 2 primarily due to greater nuclear charge of group 13 elements as compared to group 2 elements.

It depends upon the number of shells and screening effect of orbitals. ^ "Empirical Definition & Meaning - Merriam-Webster".Hint: The size of atom is defined as distance between nucleus and outermost electron.^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb S."Atomic Screening Constants from SCF Functions. ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce E."Consistent van der Waals Radii for the Whole Main Group". ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar Mantina, Manjeera Chamberlin, Adam C.^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al A.^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce cf cg ch J.C.Data derived from other sources with different assumptions cannot be compared. The radius of an atom is not a uniquely defined property and depends on the definition.Such theoretical predictions are useful when there are no ways of measuring radii experimentally, if you want to predict the radius of an element that hasn't been discovered yet, or it has too short of a half-life. Experimental data on the other hand are only based on theories. However, often the empirical results then become an equation of estimation. Although, note that the values are not calculated by a formula.
It basically means that you measured it through physical observation, and a lot of experiments generating the same results.


 0 kommentar(er)
0 kommentar(er)
